- Clinical Basics: Vault Admin
After users and the study are created, you need to specify which users should be designated as Study Personnel for the study. This process will serve to both track the study roster, as well as grant vault users study-specific access to documents and data.
To grant a user study access:
-
Navigate to the Clinical Admin > Studies.
-
Select a study.
-
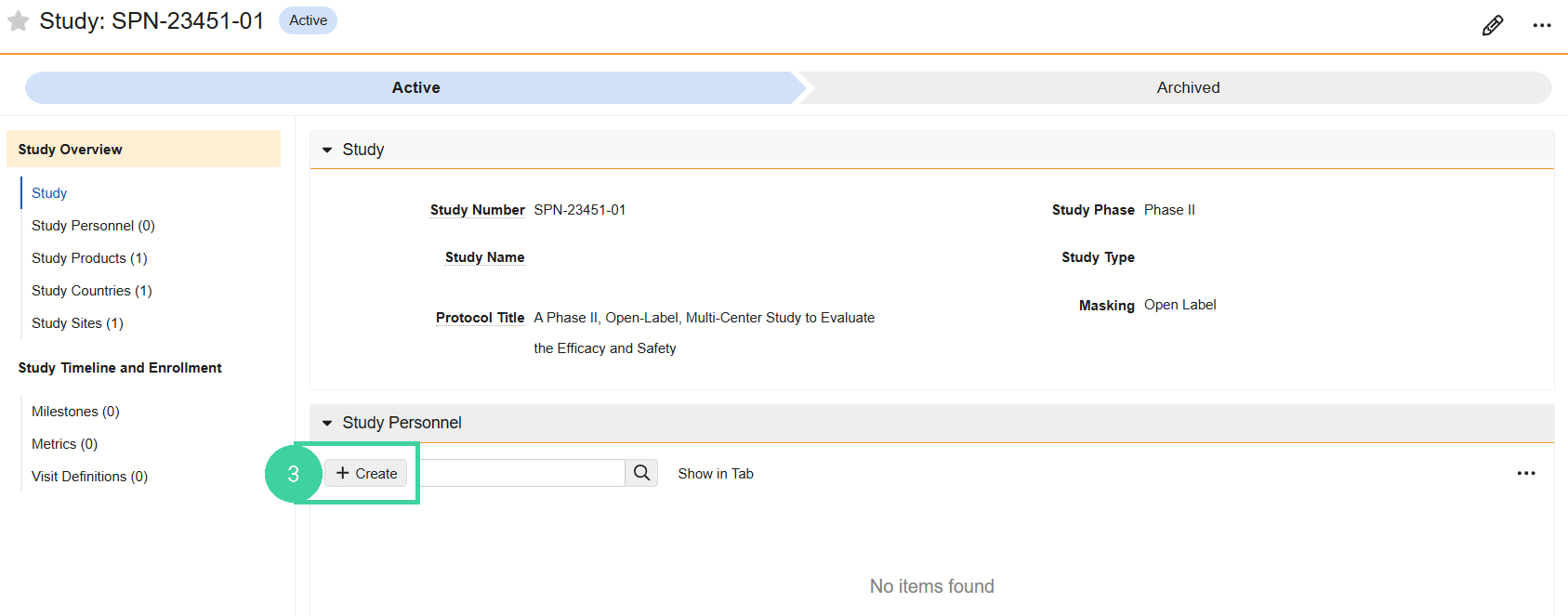
Open the Study Personnel section and click Create.
-
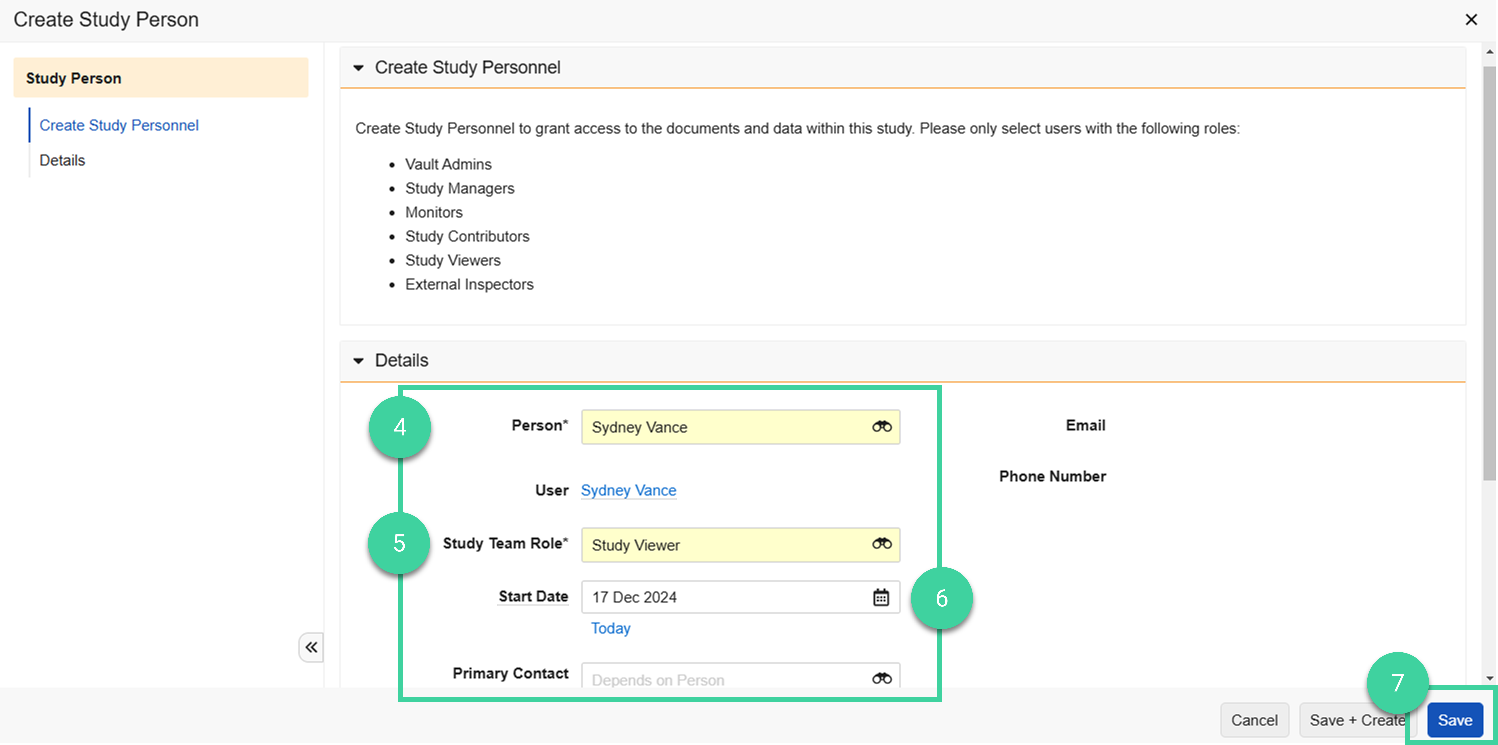
Select a Person from the dropdown.
Please only select users with the following roles:
- Vault Admins
- Study Managers
- Monitors
- Study Contributors
- Study Viewers
- External Inspectors
-
Select the person’s Study Team Role.
-
Specify a Start Date for the person.
-
Click Save.
Remove Study Access
To remove a user’s study access:
-
Navigate to the Clinical Admin > Studies select a Study.
-
Open the Study Personnel section and do one of the following:
-
Hover over the user’s name and select Edit from the All Actions menu.
-
Click the user’s name and click Edit.
-
-
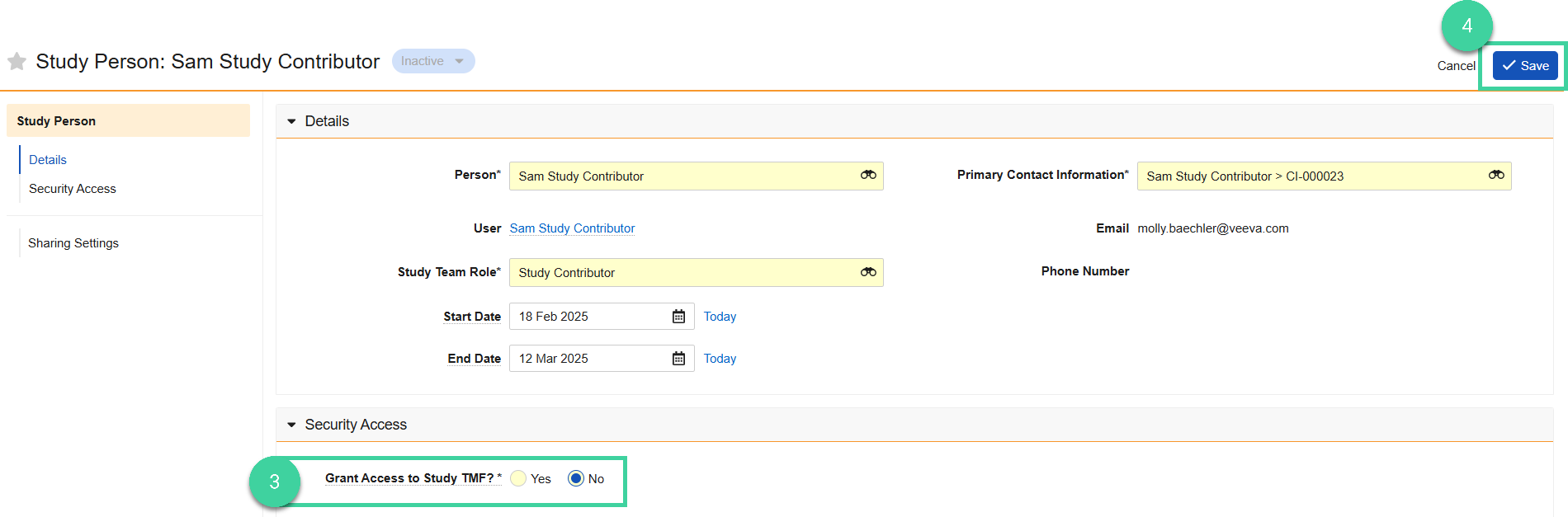
Open the Security Access section and set Grant Access to Study TMF to No.
-
Click Save.