- Clinical Basics: CTMS Vault Admin, Clinical Basics: Study Manager, Clinical Basics: Monitor
Milestones play a key part in driving the efficiency and timeliness of a study. Typically, the Study Manager will be responsible to record the planned dates, while the Monitor will be responsible to record certain actual dates.
Milestones dates are recorded throughout the study and should be kept up to date. They are typically planned and tracked at the Study level and the Site level, but can also be planned and tracked at the Country level as well.
See CTMS Milestones to learn how and when milestones are recorded.
To record milestones at the Study level:
-
Navigate to the Study Management homepage and select a Study. Do not drill down to the country or site level.
-
Click the study link.
-
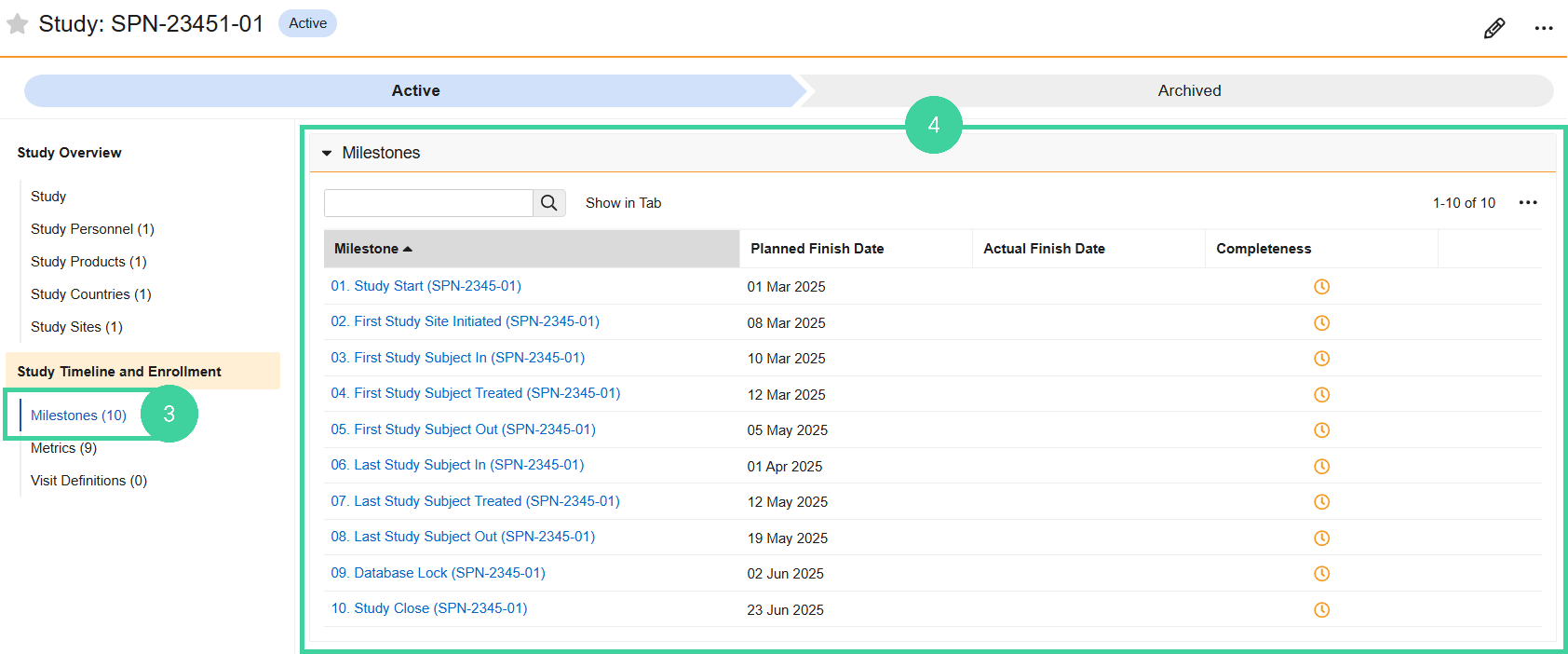
Click Milestones from the Study Timeline and Enrollment group on the left.
-
Record Planned or Actual Milestones in the grid.
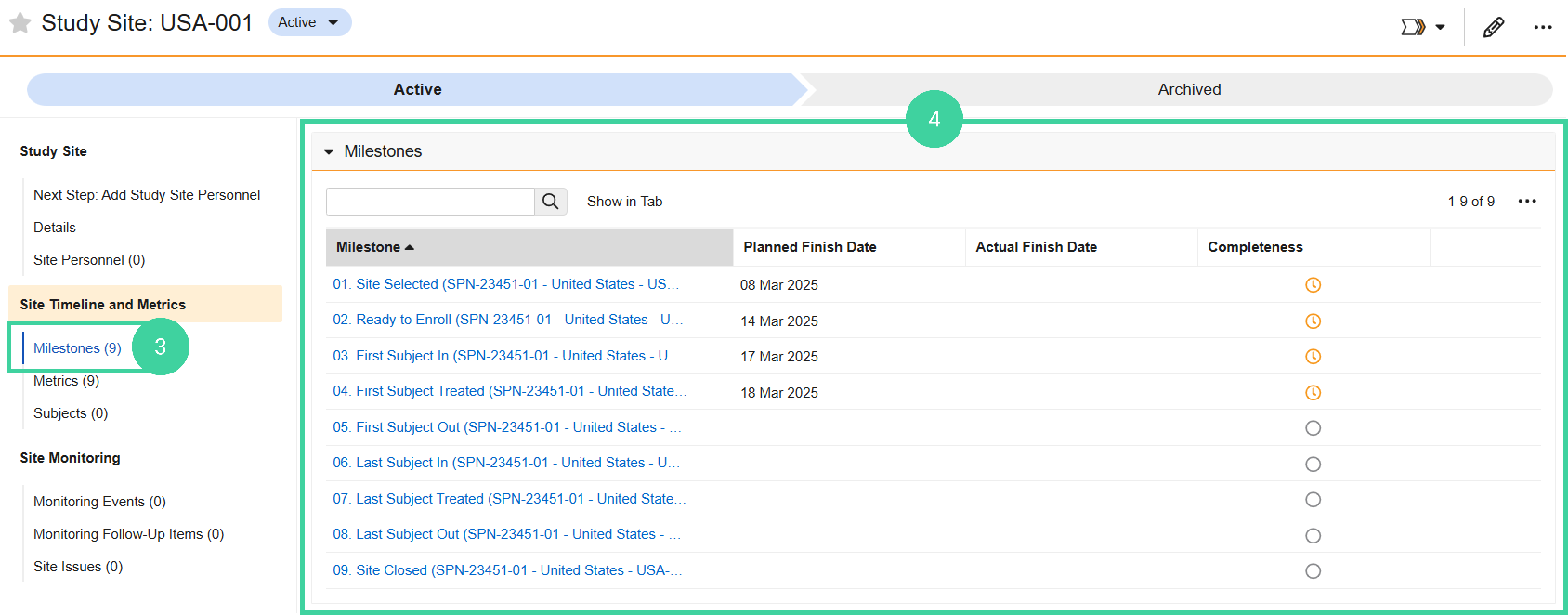
To record milestones at the Site level: